- 1 The Mycorrhizal Symbiosis
- 2 The Biology of the Ectomycorrhizal Symbiosis
- 3 Ectomycorrhiza Morphogenesis
- 4 The role of mycorrhizal fungi in nutrient cycling at the soil-root interface
- 5 The ectomycorrhizal basidiomycete Laccaria bicolor
- 6 Ecology of Ectomycorrhizal Fungi
The Mycorrhizal Symbiosis
The mycorrhizal symbiosis is undoubtedly the commonest and most important mutualistic symbiosis in the world. Simply stated, nearly all families of plants form root symbiotic organs, termed mycorrhizas, with soil fungi belonging to all the main phyla; namely Glomeromycota, Ascomycotina and Basidiomycotina. In forest, pasture and meadow soils, mycorrhizal fungi are almost ubiquitous. Their vegetative mycelium and root tips form a mutualistic association. The novel composite organ is the site of nutrient and carbon transfer between the symbionts. The various mycorrhizal associations allow terrestrial plants to colonize and grow efficiently in suboptimal environments.
There are two main types of mycorrhizal symbiosis: ectomycorrhiza (ECM) and endomycorrhiza (AM). Ectomycorrhiza, or ectotrophic mycorrhiza, are formed by fungi that are only externally associated with the plant root, whereas endomycorrhiza fungi (Glomeromycota) form their associations within the cells of the host. Another group of mycorrhizas, the ericoid mycorrhizas, are ecologically important, but mainly restricted to heathlands. While a relatively small number of plants develop ECM, they dominate forest ecosystems in boreal, temperate and mediterranean regions. In the different mycorrhizal associations, extramatrical and intraradicular networks of hyphae are active metabolic entities that provide essential nutrient resources (e.g. phosphate and amino acids) to the host plant.
These nutrient contributions are reciprocated by the provision of a stable carbohydrate-rich niche in the roots for the fungal partner, making the relationship a mutualistic symbiosis. The ecological performance of mycorrhizal fungi is a complex phenotype affected by many different genetic traits and by biotic and abiotic environmental factors. Without doubt, anatomical features (e.g., extension of the extraradical hyphae) resulting from the development of the symbiosis are of paramount importance to the metabolic (and ecophysiological) fitness of the mature mycorrhiza.
Ectomycorrhizal fungi form a mutualistic symbiosis with the majority of tree species. While a relatively small number of plants, ca 8000, form ECM, their ecological importance is amplified by their wide occupancy of biomes. Trees of Betulaceae, Cistaceae, Dipterocarpaceae, Fagaceae, Pinaceae, Myrtaceae, Salicaceae and several tribes in Fabaceae are ectomycorrhizal plants, dominating boreal, temperate, mediterranean and some subtropical forest ecosystems.
The fungi are unique in having a simultaneous dual life-style, living both within the plant roots as symbionts and, at the same time, in the soil as facultative, transitory saprotrophs. Without this symbiosis, forests as we know them could probably not exist because of the essential role of the fungi for tree growth and in the cycling of essential nutrients. Thus, ECM fungi are responsible for a symbiosis of global ecological and economic importance. The trees “feed” the ECM fungus with plant-derived carbohydrates and the fungus then utilizes this energy to decompose and assimilate essential nitrogen and phosphate compounds in the soil and transfer them back to the trees. Moreover, the association of different plants with the same guild of fungi mediates indirect inter-plant interactions, such as nutrient transfer or competition. Understanding how the fungus can achieve this is essential because of the key role of forests in buffering/sequestering increased CO2 and also for understanding how to optimize tree productivity in future biofuel production.
The Biology of the Ectomycorrhizal Symbiosis
Within days after their emergence in the upper soil profiles (e.g. organic humus and mor layer), up to 95% of the short roots of most conifers and deciduous trees are colonised by ectomycorrhizal mycobionts. The approx. 8,000 ectomycorrhizal basidiomycetes (e.g. agarics, boletes) and ascomycetes (e.g. truffles) are not a phylogenetically distinct group, but an assemblage of very different fungal species that have independently developed a symbiotic lifestyle over the last 150-180 millions years. The switch between saprotrophic and mycorrhizal lifestyles probably happened convergently, and perhaps many times, during evolution of these fungal lineages. This may have facilitated evolution of ectomycorrhizal lineages with a broad range of physiological and ecological functions reflecting partly the activities of their disparate saprotrophic ancestors. Whereas a few Basidiomycota clades are exclusively ectomycorrhizal (e.g. bolets), most clades comprise both ectomycorrhizal and saprobic species (e.g. Tricholomataceae) suggesting that the symbiotic ability involved a limited number of (symbiotic) genes.
Although it was thought that ectomycorrhizal fungi were colonizing only trees, the use of DNA-based genotyping methods has recently modified our understanding of the specificity of these fungi towards their host plants. For example, Rhizoctonia spp were reported until recently as the dominant mycorrhizal symbionts of orchids, but direct amplification of fungal DNA from mycorrhizal roots of achlorophyllous and green forest orchids demonstrated that the main symbionts are unculturable fungi that belong to known ectomycorrhizal taxa (truffles, Sebacinaceae). Therefore, the current classification of mycorrhizal associations does not take in account the plasticity of the different fungal groups in forming ectomycorrhizal interactions.
Ectomycorrhiza Morphogenesis
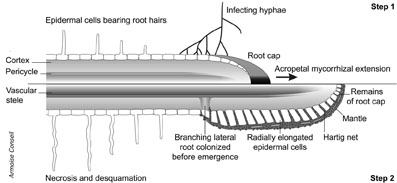
Signalling processes bring the mycobiont into the vicinity of susceptible host roots. Only the broad outlines of the signalling processes have been defined, and a limited set of chemical signals produced by either the host or symbiont have been identified so far. Early stages of ectomycorrhiza development have well-characterized similar morphological transitions. Symbiosis development proceeds through a programmed series of morphogenetic events. Fungal hyphae emerging from soil propagules (spores, sclerotia) or an older mycorrhiza penetrate into the root cap cells and grow through them. Backwards from the tip the invasion of root cap cells proceeds inwards until the hyphae reach the epidermal cells. Morphogenetic changes take place upon contact of the hyphæ with living cortical cells, which is pivotal for initiation of mantle formation and Hartig net construction. The hyphae progressing in the root apoplastic space proliferate leading to the formation of a finger-like, labyrinthine system, the so-called Hartig net. Abundant membranes of this structure allow ions, metabolites and effector molecules to pass at a high rate between adjacent cells, providing the anatomical basis for intercellular communication and the local coordination between the symbionts. Numerous mitochondria, lipid bodies, dictyosomes with proliferating cisternae, and extensive endoplasmic reticulum are contained within the coenocytic hyphæ of the Hartig net, all of which are illustrations of a highly active anabolic state with high biosynthesis of secreted proteins. Progression from the strongly rhizomorphic outgrowth of the free-living mycelium to the plectenchymatous structure of the ectomycorrhizal sheath and the coenocytic Hartig net hyphæ is associated with a lack of septation, a loss of apical coherence and intimate juxtaposition of hyphæ. No root cell penetration is observed, except in senescing ectomycorrhizal tips. After attachment onto epidermal cells, hyphae multiply to form a series of layers of several hundred µm thick which differentiates to form the mature mantle. The hyphae in these structures are encased in an extracellular polysaccharide and proteinacous matrix. Air and water channels that allow the flow of nutrients into the symbiosis innervate these structures, although most of the nutrient transfer probably takes place via the symplastic way. An outward network of hyphae prospecting the soil and gathering nutrients irradiate from the outer layers of the mantle.
The role of mycorrhizal fungi in nutrient cycling at the soil-root interface
Symbiotic roots provide a niche for mycorrhizal fungi. To bring about a symbiosis, the host plant must trade the fungus’s demand for carbon for respiration and growth, which is met primarily by glycolytic and anaplerotic processes requiring carbon sources, against its provision of extra nitrogen, phosphate and minerals. Hyphae prospecting the soil absorb nutrients by active metabolism and transport ions and assimilated metabolites to the host root via their strands and rhizomorphs. This mechanism is crucial for the absorption of nutrients that are poorly mobile, such as inorganic phosphate (Pi) and K+, or bound to soil particulates (NH4+). Since ions rapidly absorbed by non-mycorrhizal plant roots become scarce in the rhizosphere, a zone of deficiency forms and the root’s absorption rate mainly depends upon their diffusion rate rather than its own activity. Mycorrhizal hyphae counteract this deficiency, since nutrients translocate through the fungal cells to any sink, such as the root cells, more quickly than they diffuse in the soil. This faster translocation rate is sufficient to explain the enhanced absoption rates of symbiotic roots. In exchange, the fungus receives their carbon compounds. These two-way flows of nutrients and other metabolites take place when physiologically active cells of both partners are in intimate contact.
The ectomycorrhizal basidiomycete Laccaria bicolor
The symbiotic fungus Laccaria bicolor (Maire) P.D. Orton (common name: bicoloured deceiver) is a member of the Hydnangiaceae (Agaricomycotina, Agaricales), a large clade of ectomycorrhizal basidiomycetes. Laccaria is a cosmopolitan and common genus of mushroom-forming Agaricales which has been reported from numerous ectomycorrhizal plant communities. The systematics, biogeography, and ecology of Laccaria secies have therefore been well-studied (‘The Mushroom Genus Laccaria in North America’). Its physiological ecology is well known among ectomycorrhizal taxa, because it grows rapidly in pure culture and its mycorrhiza are easily established with tree roots under laboratory conditions. Finally, this species is used in large-scale commercial inoculation programs in forest nurseries worldwide to enhance growth of tree seedlings. To elucidate the genetic basis of the mycorrhizal symbiosis, the Laccaria Genome Consortium, in collaboration with the Joint Genome Institute (U.S. Department of Energy), have sequenced and analyzed the 65 million base-pair genome of Laccaria bicolor, strain S238N-H82, to high draft using a whole genome shotgun method. This is the first symbiotic fungus genome to be sequenced.
– The life cycle of Laccaria bicolor
In Laccaria bicolor, single meiospores germinate in the vicinity of host tree roots to produce haploid, monokaryotic mycelia. Two complex mating-type factors control sexual compatibility in the monokaryons and regulate the maintenance of the dikaryotic state. Fusion of sexually compatible haploid monokaryotic mycelia results in the formation of the dikaryotic (diploid) mycelium. The hyphae of Laccaria bicolor dikaryons develop clamp connections at each septum, while the hyphae of monokaryons do not. The dikaryon is the predominant vegetative structure in Laccaria bicolor and most other basidiomycetes. Under appropriate conditions, the filamentous dikaryotic mycelium attached to the host roots produces the fruiting bodies within which meiosis occurs. The monokaryotic and dikaryotic mycelia are capable of indefinite growth in soil, allowing for the maintenance and duplication of the genotype of each ploidy state. The ectomycorrhizal symbiosis is generally induced by dikaryotic mycelia interacting with short roots of the host tree.
– Laccaria bicolor S238N-H82: origin and culture conditions
A fruiting body of Laccaria bicolor was collected in 1976 under Tsuga mertensiana in the Crater Lake National Park (Oregon, USA) by J Trappe and R Molina and deposited at the Forest Service (Corvallis, OR, USA). A subculture of this strain, so-called S238-O, was transferred to the INRA-Nancy in 1980 and renamed S238N. Spores were obtained from a fruiting body collected under seedlings of Douglas fir (Pseudotsuga menziesii), inoculated with L. bicolor strain S238N in a glasshouse and germinated according to Fries. Up to 100 different monokaryotic mycelia, including the H82 line, were stored and subcultured at the Tree-Microbe Interactions Unit (INRA-Nancy). For purification of the high molecular weight DNA used for genomic library construction, the haploid monokaryotic line S238N-H82 was grown in petri dishes containing a Pachlewski-agar medium and incubated at 25°C.
Ecology of Ectomycorrhizal Fungi
Over the last decade, studies of ectomycorrhizal communities have shown the following:
- Not all ectomycorrhizal fungi produce conspicuous epigeous fruiting bodies and of those fungi that do produce conspicuous fruiting bodies, a species’fruiting body production does not necessarily reflect its below-ground abundance.
- A few fungal ECM taxa account for most of the mycorrhizal abundance and are widely spread, whereas the majority of species are only rarely encountered.
- The spatial and seasonal variations of ECM fungi is very high.
Despite the fact that mycorrhizal fungi play an important role in N, P and C cycling in ecosystems in decomposing organic nitrogen compounds, the detailed function of fungi in nutrient dynamics is still poorly known. Mycorrhizal fungi differ in their functional abilities and the different mycorrhizas they establish thus offer distinct benefits to the host plant. Some fungi may be particularly effective in scavenging organic N, and may associate with plants for which acquisition of N is crucial; others may be more effective at P uptake and transport. The formidable webs of extramatrical hyphae of mycorrhizal fungi not only permeate the mineral soil horizons, but are also very abundant in litter and decaying wood debris.
Saprotrophic fungi were found to primarily colonize relatively recently shed litter components on the surface of the forest floor, where organic C was mineralized while N was retained. Mycorrhizal fungi were prominent in the underlying, more decayed litter and humus, where they apparently mobilized N and made it available to their host plants. Mycorrhizas not only shape the plant communities, they also affect the functional diversity of rhizospheric bacteria.
Environmental factors and forest land usage could alter the composition of mycorrhizal communities.